Ted Pella公司的微波技术及方法带来了组织处理领域的一场革新。相比于传统处理方法,使用新一代组织处理系统PELCO® BioWave® Pro+及其提供的方法,不仅组织处理时间可节省高达95%,而且能始终如一地得到极佳的实验结果。
样品温度及微波辐射功率的控制是有效利用微波技术的关键。PELCO® BioWave® Pro+微波快速组织处理系统对温度和样品状态能进行精准的控制和监控,所采用的精准技术包括:
- 专利的ColdSpot™技术(美国专利号6329645)
- 真正的可变功率
- 数字化程序控制
- 内置真空及水循环系统
- 自动磁电管预热
- 外置SteadyTemp™水温控制
1、 超卓的微波控制功能
超卓的微波功率和温度控制 使样品处理结果变得更为可靠。功率大小从110至750W之间连续可调;
2、 无可比拟的应用灵活性
适用于电镜组织处理、免疫标记、脱钙、固定等各种用途;
3、 升级的方法管理程序
提供对样品处理方法的储存、编辑、调用等各种功能;
4、 真正简便的操作过程
直观的智能触摸屏控制面板使得操作过程简单,大部分设置一目了然;
5、 可靠的样品处理结果
强大的微波控制功能保证了可靠而高质量的样品处理结果;
6、 精准的样品温度控制功能
避免了样品受热的不足,充分利用了电镜样品微波处理的优势。
7、 方便快捷
Pro+组织处理系统应用范围非常广泛,只要配置相应的处理附件,就可以在一台设备上实现多种功能,主要包括:
- 电镜组织处理
- 免疫标记
- 甲醛固定及EDTA脱钙
- 石蜡组织处理
- 共焦显微镜及原位杂交
二、新特点
|
友好的运行界面
大部分的参数都直观、清晰的显示在一个屏幕中
|
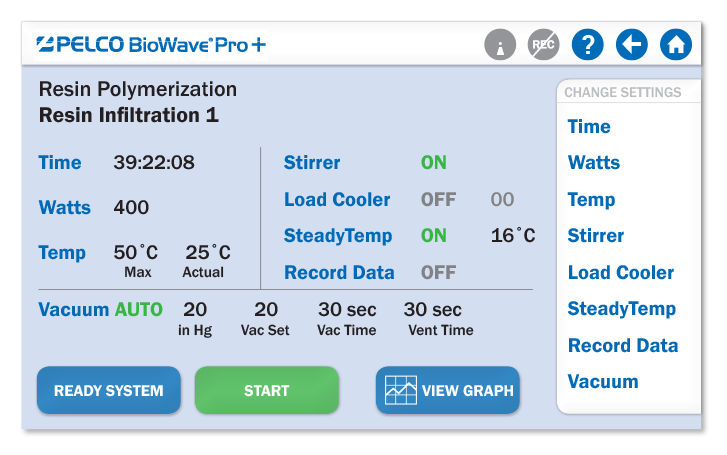 |
|
生动的运行-时间图表
可迅速便捷的查看当前执行程序的实时运行状况
|
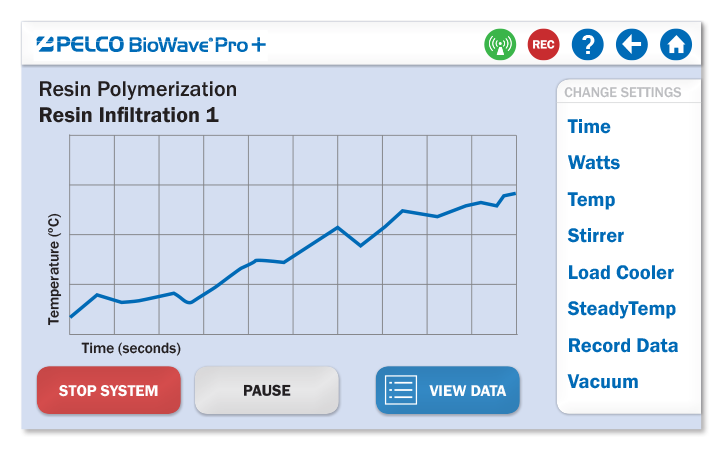 |
|
简洁的方法选择
图标化的界面让用户瞬间就可以找到所需程序
|
 |
|
全新的方法管理功能
每个用户都可以直接在设备里创建自己的文件夹,用来存放所需程序,或者将程序直接导入U盘里。因此,BioWave® Pro+非常适合中心实验室或多用户实验室。
|
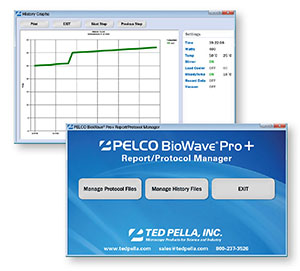 |
三、操作面板
* 手动控制
* 自动控制
* 设置
* 系统选项
四、技术参数
* 采用精准的工程技术,专为实验室而研制,微波发射器,由微机控制。
* 微波功率:100-750W之间连续可调,以1W递增,微波频率:2.45GHz
* 炉腔包裹层材料防酸、防有机试剂
* 时间设定:1秒到96小时之间可调
* 功能控制:7"友好直观视窗触摸屏人机界面,工业级触式按键
* 温度控制:±1℃(对于大多数水液),温度超出预设范围时自动停止微波辐射
* 内部冷却控制:集成的水冷系统,循环水流量:1.5L/分钟
* 外部冷却系统(选件):PELCO Steady Temp*,450W冷却系统
* 磁搅拌器:内置,0-3000rpm
* 出口:110cfm容量
* 放气:门打开时自动放气
* 样品处理方法管理:可存储多达204步的方法
* 真空系统:20"Hg,三种可选模式
* 认证:ETL/CE
* 电压要求:10A/230V
* 尺寸:553mm x 514mm x 546mm
* 重量:37kg
五、图片效果
 |
Fig. 1A-C. Figure 1A is a micrograph of a normal sural nerve with a non-myelinated nerve (N) having secretory vesicles (sv), microtubules (mt) and a swan cell nucleus (ScN). The insert (1B) shows a myelinated nerve and the arrows clearly demonstrate its periodicity. Figure 4C is a membranouse Lupus nehpritis (RPS/ISN Class V). There is diffuse, generalized effacement of the foot processes of the visceral epithelial cells. Numerous regularly disposed epimembraneous immune complex deposits are illustrated by the arrows. Both tissues were initially fixed in a variant of 10% NBF (Carson et al. 1972) and then processed in the microwave for ultrastructural evaluation by the methods of Giberson et al. (2003) and Austin (2002). Micrographs from Ronald L. Austin, Research Associate, Dept. of Pathology, LSU Medical Center, Shreveport, LA 71130.
|
 |
Figure 2A-G. Fig. 2A-B shows cytoplasmic iridovirus from the skin of a sturgeon. The iridovirus is a large enveloped dsDNA virus which infects both insect and vertebrate hosts. Fig. 2C-E demonstrates an intranuclear baculovirus from the hepatopancreas of a crayfish from Northern California. Fig. 2C is a low magnification image of the enveloped dsDNA virus showing the intranuclear arrangement of virus particles Fig. 2D is a higher magnification showing both a cross-sectional and longitudinal view of the virus. Fig. 2E is a high magnification cross-section of a number of virus particles demonstrating the unique intranuclear membrane-bound virions. Fig. 2F-G demonstrates an endothelial cell polyoma virus from a blood vessel in the liver of a parakeet. Polyoma virus have a single molecule of circular dsDNA and the particles are non-enveloped. Polyoma virions are spherical in outline and typically 45nm in diameter. Fig. 2F is a low magnification image showing typical nuclear presentation. Fig. 2G is a high magnification view of the virus. Infected tissues were processed directly from 10% NBF by the microwave methods of Nordhausen and Barr (2001) and Nordhausen et al. (2002). Micrographs from Bob Nordhausen, Univ. of California, Davis, California Animal Health and Food Safety Lab, School of Veterinary Medicine, Davis, CA 95616.
|
 |
Figure 3. Micrographs from a 2008 microwave workshop held at the Center for Microscopy, San Joaquin Delta College, Stockton, CA. Rat brain (not perfusion fixed) (1), cardiac muscle (2), kidney (3) and liver (4) were processed from osmium through resin polymerization for a net turnaround time of under 4 hours from fresh tissue to the electron microscope. Microwave techniques (Giberson, et al., 2003) make it possible to teach and in real time demonstrate the techniques of electron microscopy.
|
 |
Figure 4A-F. Figures A-C are of normal mouse liver benched fixed in 10% NBF for 24 hours. Figures D-F are of normal mouse liver fixed in 10% NBF for 20 minutes utilizing microwave radiation. All tissues were prepared for fixation identically and cut to 2mm prior to fixation. Figures A and D are corresponding Hematoxylin and Eosin stained sections and figures B and E are corresponding Vimentin IHC stained sections. Figures C and F are corresponding EM sections demonstrating complimentary ultra-structure. Images are from Dr. Jose Galvez, Center for Comparative Medicine and Department of Medical Pathology, University of California, Davis, CA.
|
 |
Figure 5. Tissues formalin fixed and paraffin processed by the protocols described in Galvez et al. 2006. Mouse mammary tumor virus induced mammary carcinoma (A, B). Note the mitotic figures (arrows) in B. Mouse esophagus with clearly identifiable muscle striations (*) (C). Mouse uterus stained with mouse anti-estrogen (D). (Center for Comparative Medicine and Department of Pathology, Univ. of Calif., Davis)
|
 |
Figure 6: Confocal projection of Elodea canadensis labeled with Hoechst 33258 nucleic acid probe (blue stain) for 6 minutes in the absence (A) and presence (B) of 150W microwave radiation. Confocal projection of Arabidopsis thaliana root tip labeled with Hoechst 33258 nucleic acid probe after 6 minutes of 150W microwave radiation (C). Confocal projection of in situ hybridization patters of whole chromosome probes (red) hybridized to nuclei of paraffin embedded rabbit skin (D). Confocal projection of mouse kidney paraffin sections labeled with anti-Factor VIII monoclonal antibody for 60 minutes on the bench (E) and after 6 minutes of 150W radiation (F) (Scale for all bars = 50µm) (Table 1). (Mark Sanders, Imaging Center, College of Biological Sciences, Univ. of Minnesota, St. Paul, MN). Reprinted with permission of Galvez et al., Microscopy and Analysis, Nov. 2006.
|
 |
Figure 7. Retinas were fixed in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) overnight at 4°C. Following fixation the tissue was rinsed 6 x 20 minutes in buffer prior to beginning antibody labeling. The bench staining protocol required 7 days. The labeling results were completed in an afternoon using microwave-enhanced labeling during a workshop held at the Univ. of Minnesota Imaging Center (Mark Sanders, Director - May 17-19, 2006). The retinas were triple-labeled for:
- Collagen Type IV (basal lamina surrounding blood vessels) with rabbit anti-type IV collagen and the secondary conjugated to FITC (green label)
- Glutamine Synthetase (enzyme found in retinal Müller glial cells) with mouse anti- glutamine synthetase and the secondary conjugated to Cy3 (red label)
- Glial Fibrillary acidic protein (GFAP an intermediate filament protein of astroglial cells) with chicken anti-glial fibrillary acidic protein and the secondary conjugated to Cy5 (blue label)
Primary antibody labeling was done at 170W for 12 minutes (4 on - 4 off - 4 on) under vacuum (15" Hg). Secondary antibody labeling was done at 170W for 6 minutes (2 on - 2 off - 2 on) under vacuum (15" Hg). Images were collected on a Nikon C1si Confocal Microscope.
|
 |
Figure 8. Note the symmetrical pattern of demineralization when microwave methods are employed. The separated piece at the top broke off during plastic embedding. From the work of Steven P. Tinling (Tinling et al., 2004), Otolaryngology Research Laboratory, University of California, Davis 95616.
|
  |
| Figure 9. Left Image. Image on the left is an electron micrograph of an inner hair cell (IHC) and supporting cells (S) from a Japanese macaque monkey. The lower left arrow indicates the region shown at higher magnification in the image to the right. Bar = 3.0µm. Right Image. In the supporting cells next to the inner hair cell, the rough endoplasmic reticulum and microtubules are well-preserved. Bar = 0.5 µm. Reprinted with permission from Madden and Henson, 1997. From a paper on microwave accelerated decalcification by Madden and Henson, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC. |
六、参考文献
- Galvez, J.J., Adamson, G., Sanders, M.A., Giberson, R.T., (2006) Microwave tissue processing techniques: their evolution and understanding. Microscopy and Analysis 20:23-24.
- Gerrity, R.G., Forbes, G.W., (2003) Microwave processing in diagnostic electron microscopy. Microsc. Today, 11(6):38-41.
- Gerrity RG, Forbes GW (2002) Microwave processing in diagnostic electron microscopy. Microsc Microanal 8(Suppl 2):152-153.
- Giberson, R.T., Austin, R.L., Charlesworth, J., Adamson, G., Herrera, G.A., (2003) Microwave and digital imaging technology reduce turnaround times for diagnostic electron microscopy. Ultrastruct. Pathol. 27:187-196.
- Wendt KD, Jensen CA, Tindall R, Katz ML, (2004) Comparison of conventional and microwave-assisted processing of mouse retinas for transmission electron microscopy. J Microsc 214:80-88
- Austin, RL (2002) The use of microwave technology in a clinical EM laboratory. Microsc Microanal 8(Suppl 2):154-155.
- Nordhausen RW, Barr BC, Hedrick RP (2002) Microwave-assisted rapid tissue processing for disease diagnosis in a veterinary diagnostic laboratory. Microsc Microanal 8(Suppl 2):150-151.
- Nordhausen RW, Barr BC (2001) Specimen preparation for this-section electron microscopy utilizing microwave-assisted rapid processing in a veterinary diagnostic laboratory. In Giberson RT, Demaree RSJr, eds. Microwave Techniques and Protocols, Totowa, NJ, Humana Press, 49-66.
- Giberson, RT., Elliott, DE (2001) Microwave-assisted formalin fixation of fresh tissue: A comparative study. In Giberson R.T., Demaree R.S.Jr., eds. Microwave Techniques and Protocols, Totowa, NJ, Humana Press, pp191-208.
- Galvez, JJ., Giberson, RT, Cardiff, RD (2006) The role of microwave radiation in reducing formaldehyde fixation times. The J. Histotechnol. 29:113-121.
- Galvez, JJ, Giberson, RT, Cardiff, RD (2004) Microwave mechanisms – the energy/heat dichotomy. Microsc. Today, 12(2):18-23.
- Munoz, TE, Giberson, RT, Demaree, R, Day JR (2004) Microwave-assisted immunostaining: a new approach yields fast and consistent results. J. Neurosci. Methods, 137:133-139.
- Sanders, M.A., Gartner, D.M. (2001) In vivo microwave-assisted labeling of Allium and Drosophila nuclei. In Giberson R.T., Demaree R.S.Jr., eds. Microwave Techniques and Protocols, Totowa, NJ, Humana Press, pp155-164.
- Madden, V.J., Henson, M.M. (1997) Rapid decalcification of temporal bones with preservation of ultrastructure. Hearing Research, 111;76-84.
- Tinling, S.P. Giberson, R.T., Kullar, R.S., (2004) Microwave exposure increases bone demineralization rate independent of temperature. J. Microsc., 215:230-235.
|